Guven Lab. on Therapeutic Bioengineering
OVERVIEW
Advances in stem cell biology and genetics with bioengineering science continuously lead breakthroughs and innovations in biotechnology. We harmonize fundamental sciences such as chemistry, biology, and physics with principles of engineering to generate innovative and effective therapeutic approaches, tissue mimicries and medical microdevices for medicine and pharmaceutical sciences. Our group focuses on bioengineering of novel native-like 3D cellular microenvironments, organ-on-a-chip platforms, and cell therapy approaches and integrates them for human health.
RESEARCH INTERESTS
Tissue and cellular microenvironment engineering: An understanding of how stem cells respond to physical and chemical stimulations from their extracellular environment is essential for utilizing the therapeutic potential of stem cells. We focus on developing novel biomaterials to construct cellular niches that can provide control over the stem cell differentiation and functionalization. We build tissues for regenerative medicine and pharmaceutical applications by designing and defining the cell-material and cell-cell interactions. Utilizing the bioengineering principles we develop bioreactor systems, tissue fabrication and assembly techniques for bottom-up and top-down tissue engineering.
Bio-mimicry organ-on-a-chip platform technologies for biomedical research: Organs-on-chips technologies provide a great platform for the investigation of fundamental mechanisms of organ physiology and disease. Such tools enable control over the physical and chemical factors of an in vitro organ models. Our group focuses on designing and engineering BioMEMS for basic research on cell physiology, in vitro disease models (e.g., neurodegenerative diseases, rare diseases, and cancer), diagnostics and pharmaceutical research.
Personalized and cellular therapies: Cell-based therapies provide unique and personalized approaches in current clinical treatments. Autologous and allogenic adult mesenchymal stem cells from bone marrow, adipose tissue, Wharton jelly and dental pulp, as well as limbal stem cells, and specialized cells (e.g., chondrocytes, hepatocytes) have been demonstrated to be effective in clinics. We develop novel therapeutic methods from bench to bedside by utilizing of cell-based therapies and intraoperative approaches in clinics.
RESEARCH HIGHLIGHTS
Biological organizations are highly hierarchical in architecture from the molecular to the macroscopic level. Such complexity in tissues is formed by building units that regulate and provide the system function. To mimic and bioengineer functional tissues, we need biomanufacturing tools that can generate and manipulate multi-scale building blocks. Bottom-up tissue engineering approaches focus on creating tissues by assembling heterogeneous building units such as biomacromolecules, cells, and cell-loaded microscale hydrogels in a multiscale manner. In our recent works, we demonstrated different cell and tissue assembly approaches utilizing acoustic, magnetic, micro robotic and bioprinting technologies to generate model platforms and tissues. Bioengineering approaches are essential for current therapeutic and diagnostic applications in medicine. Together with advances in genetics and cell biology, bioengineering tools provide key elements for novel and innovative medical technologies for human health.
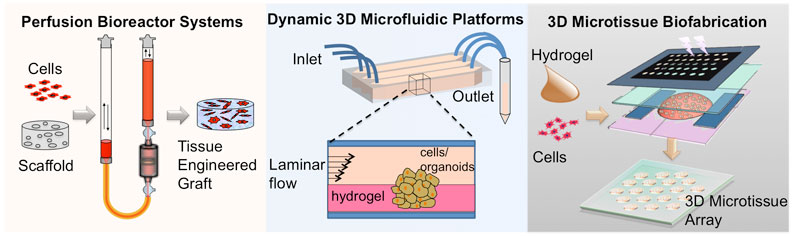
Engineering platforms and systems for biofabrication of 3D tissues and microenvironments.

Representative bioengineered tissue constructs A. Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue (Guven et al. 2011), B. In vitro endothelial capillaries from stromal vascular fraction (Guven et al. 2011), C. Enhanced engraftment and functionality of bio-engineeredautologous dermo-epidermal skin grafts pre-vascularized with adipose-derived cells (Klar et al. 2014), D. Direct neural differentiation of mouse embryonic stem cells in 3D microenvironments, E. Biotunable acoustic node assembly of hepatic organoids (Chen et al. 2015), F. Guided and magnetic self-assembly of tunable magnetoceptive cardiac microtissues (Tasoglu et al. 2014).
Group Members

Guven Lab. on Therapeutic Bioengineering
Research Group Leader
Sinan GÜVEN
sinan.guven@ibg.edu.tr
+90 232 299 41 00
(5131)
+9
0 232 299 41 63

Canan Aslı YILDIRIM
Research Group Member
asli.yildirim@ibg.edu.tr

Özge SÜER
PhD Student
ozge.suer@ibg.edu.tr

Burak KAHVECİ
PhD Student
burak.kahveci@ibg.edu.tr

Tuğba TOPBAŞ
Researcher
tugba.topbas@ibg.edu.tr

Hülya ERBOĞA
PhD Student
hulya.erboga@ibg.edu.tr

Ezgi ERDOĞAN
MSc Student
ezgi.erdogan@ibg.edu.tr

Ali Eren EVRANOS
MSc Student
alieren.evranos@ibg.edu.tr

Sultan Hilal SOLAK
Undergraduate Student
hilal.solak@ibg.edu.tr

Mustafa TİTİZ
PhD Student
mustafa.titiz@ibg.edu.tr

İlayda KOÇHAN
MSc Student
ilayda.kochan@ibg.edu.tr

Aleyna DEMİR
MSc Student
aleyna.demir@ibg.edu.tr

Gaye Su YİĞİT
MSc Student
gaye.yigit@std.ibg.edu.tr

Semih Numan GÖÇMEZ
MSc Student
semih.gocmez@ibg.edu.tr

Beyza AKTAŞ
MSc Student
beyza.aktas@std.ibg.edu.tr

Cem Batu ÖREN
Undergraduate Student
cem.oren@std.ibg.edu.tr

Şeyma AKGÜL
MSc Student
seyma.akgul@std.ibg.edu.tr
Former Members

Tuğba TOPBAŞ
Research Technician
tugba.topbas@ibg.edu.tr

Münire EKMEKÇİGİL
Post-Doc Researcher
munire.ekmekcigil@ibg.edu.tr

Emine KAHRAMAN
MSc Student
emine.kahraman@msfr.ibg.edu.tr

Elifsu POLATLI
PhD Student
elifsu.polatli@ibg.edu.tr

Gizem AKTUĞ
PhD Student
gizem.aktug@msfr.ibg.edu.tr

Olcay AKBULUD
Post-Doc Researcher
olcay.akbulud@msfr.ibg.edu.tr

Melis ASAL
MSc Student
melis.asal@msfr.ibg.edu.tr

Ali Kemal BAŞ
MSc Student
kemal.bas@msfr.ibg.edu.tr

İbrahim Halilullah ERBAY
Visiting Student
ibrahimhalilullah.erbay@msfr.ibg.edu.tr

Hanife GÖKKAN
Undergraduate Student
hanife.gokkan@ibg.edu.tr

İrem DUMAN
MSc Student
irem.duman@ibg.edu.tr

Alperen YILMAZ
Undergraduate Student
alperen.yilmaz@msfr.ibg.edu.tr

Gamze KOÇAK
PhD Student
gamze.kocak@ibg.edu.tr

Vedat SARI
MSc Student
vedat.sari@ibg.edu.tr

Ali Can KOÇ
PhD Student
alican.koc@ibg.edu.tr

Sude UYULGAN
MSc Student
sude.uyulgan@ibg.edu.tr

Merve TÜREMEN
PhD Student
merve.turemen@ibg.edu.tr

Eylem HIRLI
Undergraduate Student
eylem.hirli@msfr.ibg.edu.tr

Merve Beril DOĞDAŞ BAŞCILAR
MSc Student
merve.dogdas@msfr.ibg.edu.tr

Yusuf Çağlar ODABAŞI
Undergraduate Student
yusuf.odabasi@msfr.ibg.edu.tr

Asude SEZER
Undergraduate Student
asude.sezer@std.ibg.edu.tr

Sude ŞARU
Undergraduate Student
sude.saru@ibg.edu.tr

Gamze KOÇAK
PhD Student
gamze.kocak@ibg.edu.tr

Clara SCHIMMER
MSc Student
clara.schimmer@ibg.edu.tr

Gülşah SUNAL
MSc Student
gulsah.sunal@ibg.edu.tr

Halime GESEN
Undergraduate Student
halime.gesen@ibg.edu.tr

Ali Eren EVRANOS
Researcher
alieren.evranos@ibg.edu.tr

Ezgi ERDOĞAN
MSc Student
ezgi.erdogan@ibg.edu.tr

Buse SEZER
MSc Student
None

Ali Eren EVRANOS
MSc Student
alieren.evranos@ibg.edu.tr

Gaye Su YİĞİT
MSc Student
gaye.yigit@std.ibg.edu.tr

Sevgi KÜTÜK
Undergraduate Student
None

Mustafa Batuhan YEL
Visiting Student
None
Selected Publications
Zhu Y, Serpooshan V, Wu S, Demirci U, Chen P, Güven S. Tissue Engineering of 3D Organotypic Microtissues by Acoustic Assembly. Methods Mol Biol. 2017 September : 1-12. doi:10.1007/7651_2017_68. Download
Calibasi Kocal G, Güven S, Foygel K, Goldman A, Chen P, Sengupta S, Paulmurugan R, Baskin Y, Demirci U. Dynamic Microenvironment Induces Phenotypic Plasticity of Esophageal Cancer Cells Under Flow. Scientific reports. 2016 December ; 6 : 38221. doi:10.1038/srep38221. Download
Bouyer C, Chen P, Güven S, Demirtaş TT, Nieland TJ, Padilla F, Demirci U. A Bio-Acoustic Levitational (BAL) Assembly Method for Engineering of Multilayered, 3D Brain-Like Constructs, Using Human Embryonic Stem Cell Derived Neuro-Progenitors. Advanced materials (Deerfield Beach, Fla.). 2016 January ; 28 (1) : 161-7. doi:10.1002/adma.201503916. Download
Arslan-Yildiz A, El Assal R, Chen P, Guven S, Inci F, Demirci U. Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication. 2016 March ; 8 (1) : 014103. doi:10.1088/1758-5090/8/1/014103. Download
Saxer F, Scherberich A, Todorov A, Studer P, Miot S, Schreiner S, Güven S, Tchang LA, Haug M, Heberer M, Schaefer DJ, Rikli D, Martin I, Jakob M. Implantation of Stromal Vascular Fraction Progenitors at Bone Fracture Sites: From a Rat Model to a First-in-Man Study. Stem cells (Dayton, Ohio). 2016 December ; 34 (12) : 2956-2966. doi:10.1002/stem.2478. Download
Namkoong B, Güven S, Ramesan S, Liaudanskaya V, Abzhanov A, Demirci U. Recapitulating cranial osteogenesis with neural crest cells in 3-D microenvironments. Acta biomaterialia. 2016 February ; 31 : 301-311. doi:10.1016/j.actbio.2015.12.004. Download
Durmus NG, Tekin HC, Guven S, Sridhar K, Arslan Yildiz A, Calibasi G, Ghiran I, Davis RW, Steinmetz LM, Demirci U. Magnetic levitation of single cells. Proceedings of the National Academy of Sciences of the United States of America. 2015 July ; 112 (28) : E3661-8. doi:10.1073/pnas.1509250112. Download
Guven S, Chen P, Inci F, Tasoglu S, Erkmen B, Demirci U. Multiscale assembly for tissue engineering and regenerative medicine. Trends in biotechnology. 2015 May ; 33 (5) : 269-279. doi:10.1016/j.tibtech.2015.02.003. Download
Luo Z, Guven S, Gozen I, Chen P, Tasoglu S, Anchan RM, Bai B, Demirci U. Deformation of a single mouse oocyte in a constricted microfluidic channel. Microfluidics and nanofluidics. 2015 October ; 19 (4) : 883-890. doi:10.1007/s10404-015-1614-0. Download
Tasoglu S, Yu CH, Liaudanskaya V, Guven S, Migliaresi C, Demirci U. Magnetic Levitational Assembly for Living Material Fabrication. Advanced healthcare materials. 2015 July ; 4 (10) : 1469-76, 1422. doi:10.1002/adhm.201500092. Download
Chen P, Güven S, Usta OB, Yarmush ML, Demirci U. Biotunable acoustic node assembly of organoids. Advanced healthcare materials. 2015 September ; 4 (13) : 1937-43. doi:10.1002/adhm.201500279. Download
Kaempfen A, Todorov A, Güven S, Largo RD, Jaquiéry C, Scherberich A, Martin I, Schaefer DJ. Engraftment of Prevascularized, Tissue Engineered Constructs in a Novel Rabbit Segmental Bone Defect Model. International journal of molecular sciences. 2015 June ; 16 (6) : 12616-30. doi:10.3390/ijms160612616. Download
Guven S, Lindsey JS, Poudel I, Chinthala S, Nickerson MD, Gerami-Naini B, Gurkan UA, Anchan RM, Demirci U. Functional maintenance of differentiated embryoid bodies in microfluidic systems: a platform for personalized medicine. Stem cells translational medicine. 2015 March ; 4 (3) : 261-8. doi:10.5966/sctm.2014-0119. Download
Tasoglu S, Diller E, Guven S, Sitti M, Demirci U. Untethered micro-robotic coding of three-dimensional material composition. Nature communications. 2014 January ; 5 : 3124. doi:10.1038/ncomms4124. Download
Tasoglu S, Yu CH, Gungordu HI, Guven S, Vural T, Demirci U. Guided and magnetic self-assembly of tunable magnetoceptive gels. Nature communications. 2014 September ; 5 : 4702. doi:10.1038/ncomms5702. Download
Chen P, Luo Z, Güven S, Tasoglu S, Ganesan AV, Weng A, Demirci U. Microscale assembly directed by liquid-based template. Advanced materials (Deerfield Beach, Fla.). 2014 September ; 26 (34) : 5936-41. doi:10.1002/adma.201402079. Download
Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, Meuli M, Martin I, Scherberich A, Reichmann E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials. 2014 June ; 35 (19) : 5065-78. doi:10.1016/j.biomaterials.2014.02.049. Download
Tasoglu S, Kavaz D, Gurkan UA, Guven S, Chen P, Zheng R, Demirci U. Paramagnetic levitational assembly of hydrogels. Advanced materials (Deerfield Beach, Fla.). 2013 February ; 25 (8) : 1137-43, 1081. doi:10.1002/adma.201200285. Download
Helmrich U, Di Maggio N, Güven S, Groppa E, Melly L, Largo RD, Heberer M, Martin I, Scherberich A, Banfi A. Osteogenic graft vascularization and bone resorption by VEGF-expressing human mesenchymal progenitors. Biomaterials. 2013 July ; 34 (21) : 5025-35. doi:10.1016/j.biomaterials.2013.03.040. Download
Güven S, Karagianni M, Schwalbe M, Schreiner S, Farhadi J, Bula S, Bieback K, Martin I, Scherberich A. Validation of an automated procedure to isolate human adipose tissue-derived cells by using the Sepax® technology. Tissue engineering. Part C, Methods. 2012 August ; 18 (8) : 575-82. doi:10.1089/ten.TEC.2011.0617. Download
Mehrkens A, Saxer F, Güven S, Hoffmann W, Müller AM, Jakob M, Weber FE, Martin I, Scherberich A. Intraoperative engineering of osteogenic grafts combining freshly harvested, human adipose-derived cells and physiological doses of bone morphogenetic protein-2. European cells & materials. 2012 September ; 24 : 308-19. Download
Güven S, Mehrkens A, Saxer F, Schaefer DJ, Martinetti R, Martin I, Scherberich A. Engineering of large osteogenic grafts with rapid engraftment capacity using mesenchymal and endothelial progenitors from human adipose tissue. Biomaterials. 2011 September ; 32 (25) : 5801-9. doi:10.1016/j.biomaterials.2011.04.064. Download
Papadimitropoulos A, Scherberich A, Güven S, Theilgaard N, Crooijmans HJ, Santini F, Scheffler K, Zallone A, Martin I. A 3D in vitro bone organ model using human progenitor cells. European cells & materials. 2011 May ; 21 : 445-58; discussion 458. Download
Müller AM, Mehrkens A, Schäfer DJ, Jaquiery C, Güven S, Lehmicke M, Martinetti R, Farhadi I, Jakob M, Scherberich A, Martin I. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. European cells & materials. 2010 March ; 19 : 127-35. Download
Total : 24
Open Positions
PhD and MSc Positions in Therapeutic Bioengineering Group
Izmir Biomedicine and Genome Center Therapeutic Bioengineering Group is seeking for PhD and MSc student candidates to work on funded TUBITAK 1003 projects.
Our group harmonizes fundamental sciences such as chemistry, biology, and physics with principles of engineering to generate innovative and effective therapeutic approaches, tissue mimicries and medical microdevices for medicine and pharmaceutical sciences. The focus of the group is to bioengineer novel native-like 3D cellular microenvironments, organ-on-a-chip platforms, and cell therapy approaches and to translate them into clinics.
Candidates should hold background or prior research experience in related fields such as biomedical engineering, tissue engineering, biomaterials, BioMEMS, microfluidics, stem cells and plant cell culture. Students graduated from departments of engineering (bioengineering, biomedical, mechanical, electrical, chemical) or basic sciences (molecular biology and genetics, biology, chemistry, physics) are welcomed to apply.
Applicants with strong academic record, English skills, self-motivation and dedication to a career in science/discovery are encouraged to contact Assoc. Prof. Sinan Güven (sinan.guven@ibg.edu.tr), http://ibg.edu.tr/research-programs/groups/guven-lab/.
Awards
- Incentive Award by TUSEB (Health Institutes of Turkey), 2018
- Above-Threshold Award by TUBITAK, 2018
- Young Scientist (BAGEP) Award by Science Academy, 2017
- Above-Threshold Award by TUBITAK, 2017
- Young Investigator (GEBİP) Awards by Turkish Academy of Sciences (TÜBA), 2016
- 2232 Yurda Dönüş Araştırmacı Dolaşım Programı by TUBITAK, 2015
- Young Investigator by Orthopaedic Research Society, 2015
Academic Memberships
- TÜBA-Turkish Academy Of Science, 2016
- The Science Academy, Turkey, 2017
Contact

Guven Lab. on Therapeutic Bioengineering
Research Group Leader
Sinan GÜVEN
sinan.guven@ibg.edu.tr
+90 232 299 41 00
(5131)
+9
0 232 299 41 63